Ultra-low gossypol cottonseed (ULGCS) as a feed for non-ruminants to enhance human nutrition security
By Keerti S. Rathore1, Thomas C. Wedegaertner2, and Kater Hake2
1Institute for Plant Genomics & Biotechnology, Dept. of Soil & Crop Sciences, Texas A&M University, College Station, TX 77843, USA.
2Cotton Incorporated, Cary, NC, USA.
Introduction
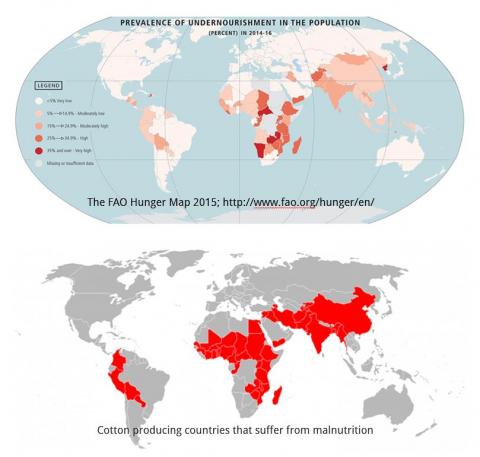
The cotton plant produces the greatest volume and most important natural fibre in the world. It has been cultivated for its fibre for over 7,000 years. Despite the availability of synthetic alternatives, it continues to serve as an important source of fibre for textiles. Cotton, adapted to warm climates, is grown in over 80 countries and is a cash crop for nearly 20 million farmers. Most of the cotton producing countries in Asia and Africa also suffer from malnutrition (Figure 1).
Figure 1: Most of the cotton producing countries suffer from undernourishment. Source: Keerti S. Rathore
An attribute of cotton not widely recognized is that for every 1 kg of fibre, the plant produces ~1.65 kg of seed. In the year 2014, global cottonseed production reached 47 million metric tons (MMT) (FAO Production Statistics). This makes cotton the third largest field crop in terms of edible oilseed tonnage in the world. In addition to 21% oil, cottonseed is a source of relatively high-quality protein (23%). Thus, global production, containing nearly 10.8 MMT of protein, can potentially provide the protein requirements of ~590 million people per year at a rate of 50 g protein/day. However, the ability to use this nutrient-rich resource for food or even as feed for monogastric animals is hampered by the presence of a toxic terpenoid, gossypol, that is unique to the tribe Gossypieae.
Terpenoids are a class of secondary metabolites that are produced by many plant species. These compounds play an important ecological role either as attractants (e.g. linalool) or as defense compounds (e.g. bitter triterpenoid cucurbitacins, pungent diterpenoid polygodial, gossypol and related compounds in cotton) (Aharoni et al., 2005; Langenheim, 1994; Stipanovic et al., 1999). However, the presence of some of the terpenoids or other types of defense compounds also renders the plants or their parts that produce them toxic to humans and animals. Over the course of human history, man has learned to either avoid consumption of toxic plants/their parts or to inactivate/neutralize the toxic compounds present before ingestion (i.e., cassava, kidney beans). In some cases, the plant product is used as feed for domestic animals with a rumen where the toxin can be inactivated or metabolized before digestion; thus the animal suffers little or no ill effect from the toxin. Gossypol-containing cottonseed, produced in abundance as a byproduct of the fibre production, represents such a case.
Gossypol is a terpenoid produced in pigment glands of plants belonging to the genus Gossypium of the family Malvaceae. Gossypol and related terpenoids are present throughout the cotton plant in the glands of foliage, floral organs, bolls, roots and seeds (Stanford and Viehoever, 1918). Constitutive presence of these compounds protects the plant from both insects and pathogens (Hedin et al., 1992; Stipanovic et al., 1999) and they are also induced in response to microbial infections as well as insect herbivory (Bell et al., 1975; Bezemer et al., 2004; McAuslane and Alborn, 1998). Gossypol causes heart and liver damage in monogastric animals including humans (Gadelha et al., 2014; Risco and Chase, 1997). Gossypol poisoning has been reported in several species, including pigs (Haschek et al., 1989), broiler chicks (Henry et al., 2001), sheep (Morgan et al., 1988), and goats (East et al., 1994). Monogastric animals, such as pigs, birds, fish, and rodents, are more susceptible to gossypol toxicity than ruminants (Kenar, 2006; Randel et al., 1992; Zhang et al., 2007). Signs of acute gossypol toxicity in most animals include impaired body weight gain, weakness, anaemia, respiratory distress, anorexia, apathy, heart failure and death after several days (East et al., 1994; Haschek et al., 1989; Henry et al., 2001; Morgan et al., 1988). Adult ruminant animals are able to tolerate a limited amount of gossypol in their diets because gossypol is bound during ruminal fermentation and becomes unavailable for intestinal absorption. Therefore, cottonseed is currently used mainly as feed for ruminant animals as either whole seed or cottonseed meal after oil extraction (gossypol is chemically and physically removed from the oil and refined oil has an important role in human nutrition). However, even adult cattle can suffer from gossypol toxicity above a certain amount of cottonseed intake (Smalley and Bicknell, 1982). Young animals, without fully developed rumen, are more sensitive to gossypol compared to the adult ruminants (Holmberg et al., 1988).
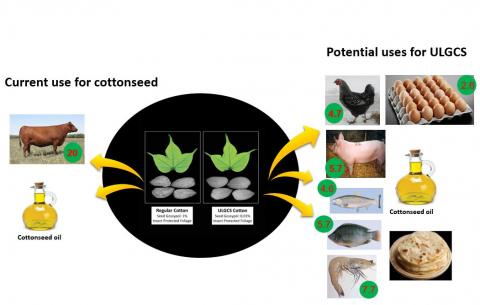
Currently, whole cottonseed or the protein-rich CSM is fed to adult ruminants that are highly inefficient in converting feed protein into meat protein. The protein conversion ratio (PCR) for beef cattle is 20 (20 pounds of crude protein in the feed converted to one pound of beef protein), while PCR for swine, chicken, tilapia, salmon, shrimp, and eggs is 5.7, 4.7, 5.7, 4.6, 7.7, and 2.6, respectively (Tilman and Clark, 2014; Boyd, 2005). If gossypol is eliminated from the cottonseed, then cotton-producing countries with limited supply of feed protein can derive greater benefits by utilizing the seed-derived protein as a feed for poultry, swine or aquaculture species.
Attempts based on plant breeding and processing
Given the toxicity of gossypol, its elimination from the cottonseed has been a long-standing goal of geneticists and cottonseed processors. The “glandless mutant cotton” cultivated by Native Americans in the Hopi region of Arizona and discovered by breeders in the 1950s was free of glands and, therefore, gossypol-free. The discovery of the glandless cotton mutant and subsequent introgression of this trait into commercial cultivars by breeders generated a great deal of excitement and provided hope for the utilization of glandless cottonseed as feed for monogastric animals and for human food (Lusas and Jividen, 1987). From 1960s through 1980s, several investigations were launched world-wide to assess the fitness of gossypol-free cottonseed as a feed for monogastric animals and even as a source of protein for human nutrition. Some studies found that processed glandless cottonseed meal (CSM) was of equal nutritional value to soybean meal (SBM) in supporting chick growth and can also be used to replace part or all of SBM in practical diets for broilers (Jonston and Watts, 1964; Waldroup et al., 1968). In a separate study, glandless CSM with SBM, supplemented with lysine and methionine, was compared to examine the performance of laying hens and egg characteristics. The results suggested that the protein of glandless CSM was about equal to SBM in sustaining the performance of the laying hens (Roberson 1970). Reid et al. (1984) found that egg production rates with diets containing up to 10% glandless CSM were comparable to those of birds fed on a SBM based diet. LaRue et al. (1985) conducted growth trials with 28-day-old, weaner pigs (7.5 kg) and growing-finishing pigs (19 to 97 kg). In these trials, glandless CSM was substituted in 20% increments for supplemental protein provided by SBM in corn-soybean based diets. Lysine was added to all glandless CSM diets to make them equal to the control corn-soybean diets. Pigs that were fed up to 40% supplemental glandless CSM protein showed similar performance to those on control, corn-soybean diet. The authors concluded that glandless CSM could be effectively used in the diets of starter, grower and finisher pigs when used in limited amounts with the addition of supplemental lysine. Glandless CSM was used not just for animal feeding studies, but was also found suitable for human nutrition (Alford et al., 1996; Bressani, 1965; Lusas and Jividen, 1987; Rathore et al., 2008).
Unfortunately, due to the lack of the glands and, therefore, the protective terpenoids in the vegetative and floral parts of the plant, glandless cotton varieties suffered more severe pest damage from traditional and also non-traditional cotton pests and had lower yields under field conditions (Bottger et al., 1964; Jenkins et al., 1966; Lukefahr et al., 1966; Maxwell et al., 1965). Thus, although the glandless cottonseed proved fit as a source of food and feed, it was not widely accepted by cotton growers. The glandless cotton experience underscored the importance of maintaining the protective terpenoids in the vegetative and floral parts of the plant. The efforts of traditional plant breeders to eliminate gossypol from the cottonseed while maintaining the normal levels of gossypol and related terpenoids in the rest of the plant have been unsuccessful. During the 1960s and 1970s several solvent removal technologies were developed, since gossypol can be removed from cottonseed meal with various solvents such as methanol, acetone and isopropyl alcohol. These solvents were investigated as a means of producing a low gossypol product that could be safely fed to pigs and chickens. For various reasons the solvent-based removal of gossypol has never been economically viable, even though the de-gossypolized meal was suitable for most feeding applications.
Biotechnology to the rescue
Failure of breeding efforts and solvent removal technologies prompted many scientists around the world, in the 1990s, to utilize the tools of advanced biotechnology to solve the problem of gossypol reduction/elimination from the cottonseed only. While many of the biochemical steps in the synthesis of gossypol were known, the first gene, that encodes an important enzyme involved at a critical step in gossypol biosynthetic pathway, was identified and cloned in 1995 from an old world diploid cotton species (Chen et al., 1995). The sequence information was used to fish out the same gene from the most widely grown tetraploid cotton (Sunilkumaret al., 2006). In addition, another important component, a DNA sequence that can be used to control seed-specific expression or silencing of a given gene was also isolated from cotton and characterized (Sunilkumaret al., 2002). In the late 90s, the biological community came to understand a natural biological phenomenon known as RNA interference (RNAi) that can also be used to silence a desired gene in an eukaryotic organism. Our team at Texas A&M University used a combination of these three tools and technologies to engineer a cotton plant that resulted in the reduction of gossypol from ~10,000 ppm to about 250 ppm in the seed (the United Nations Food and Agriculture Organization and World Health Organization guidelines permit up to 600 ppm free gossypol in edible cottonseed products). Importantly, the levels of gossypol and related protective terpenoids that are derived from the same biosynthetic pathway were not diminished in the foliage and floral parts of the mature plants and thus remain available for plant defense against insects, predation and diseases. The stability of this important trait has been confirmed by evaluation of several Ultra-low Gossypol Cottonseed (ULGCS) lines under field conditions.
The results obtained from greenhouse and field trials show that the ULGCS lines do not suffer any penalty in terms of agronomic performance or fibre/seed yield and quality. The foliage, floral parts and the roots of the plant retain their naturally occurring, chemical defense compounds including gossypol. Thus, ULGCS overcomes the major weakness of the glandless cotton. Seed compositional analysis did not show major differences between the ULGCS and commercial cottonseeds except for their gossypol content. The ULGCS, being a transgenic product, is awaiting regulatory approval from two different federal agencies in the U.S., i.e. USDA-APHIS and FDA. Following U.S. regulatory approval, the technology will be shared internationally for humanitarian purposes.
ULGCS as feed for non-ruminants
Currently, even the countries that suffer from protein malnutrition and feed shortages utilize the cottonseed or CSM as a feed for ruminant animals. It is a practice that is centuries old, and understandably there will be some cultural resistance to the use of gossypol-free cottonseed in food products, even in the countries that suffer most from malnourishment. The PCR values for many monogastric farm animals, described earlier, suggest that these animals are significantly more efficient in converting plant protein into high-quality meat protein. Especially, egg and broiler production could become the most efficient use of any available feed protein source, including the ULGCS. For example, a country like India, the biggest cotton producer is also experiencing increasing consumption of eggs and poultry. A recent report (Sasidhar and Suvedi, 2015) estimates an annual growth rate of 5.57% and 11.44% in egg and broiler production, respectively, in this country that until recently used to be largely vegetarian. With the rising middle class in China, egg production rose at an incredible annual growth rate of 21.9% between 1985 and 2005 (Bingsheng and Yijun, 2008). Poultry industry, and thus the demand for feed, is likely to keep growing in several poor countries as their middle class population grows. According to a World Bank Report (no. 83177-GLB, 2013), aquaculture is the fastest growing food production system at present that is increasing at a rate of 8% per year. By the year 2030, aquaculture is projected to supply over 60% of the fish for direct human consumption. At the same time, the supplies of fishmeal and fish oil are likely to decrease and it is expected that their prices will rise by 90% and 70%, respectively. ULGCS could easily meet part of this rising demand for fish feed. The potential of ULGCS as a fishmeal replacement in the diets of shrimp and juvenile Southern flounder has been demonstrated recently (Richardson et al., 2016; Alam et al., unpublished). Additional aquaculture and poultry feeding studies are planned to fully evaluate the nutritional value of ULGCS.
Figure 2: More efficient and diversified uses of Ultra-low Gossypol Cottonseed (ULGCS) compared to the current use of cottonseed. The numbers within the green circles are protein conversion ratios (feed pro-tein used/edible animal protein produced). Source: Keerti S. Rathore.
ULGCS has the potential to add value to the seed and help mitigate protein shortages, as the increase in population and standard of living put pressure on protein supplies. Thus, a world-wide adoption of ULGCS technology will not only help address the shortages of feed protein, but will also help improve rural economies of cotton-producing nations by increasing the value of cottonseed because of its highly diversified uses.
The views expressed in this article are of the authors.
References
- Aharoni, A.; Jongsma, M. A.; Bouwmeester, H. J. (2005) Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 10: 594-602.
- Alford, B.; Liepa, G.; Vanbeber, A. (1996) Cottonseed protein: What does the future hold? Plant Foods Hum. Nutr. 49: 1-11.
- Bell, A. A.; Stipanovic, R. D.; Howell, C. R.; Fryxell, P. A. (1975) Antimicrobial terpendoids of Gossypium: Hemigossypol, 6-methoxyhemigossypol and 6-deoxyhemigossypol. Phytochemistry 14: 225-231.
- Bezemer, T. M.; Wagenaar, R.; Van Dam, N. M.; Van Der Putten, W. H.; Wackers, F. L. (2004) Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. Journal of Chemical Ecology 30: 53-67.
- Bingsheng, K.; Yijun, H. (2008) Poultry sector in China: structural changes during the past decade and future trends. In: Poultry in the 21st Century. Avian Influenza and Beyond Bangkok, November 2007, Thieme O, Pilling D (Eds.) (Food and Agriculture Organization of the United Nations, Rome). http://www.fao.org/ag/AGAInfo/home/events/bangkok2007/docs/part1/1_3.pdf
- Bottger, G. T.; Sheehan, E. T.; Lukefahr, M. J. (1964) Relation of gossypol content of cotton plants to insect resistance. J. Econ. Entomol. 57:283-285.
- Boyd, C. E. (2005) Feed Efficiency Indicators for Responsible Aquaculture. GLOBAL AQUACULTURE ADVOCATE (December): 73-74.
- Bressani, R. (1965) The use of cottonseed protein in human foods. Food Tech. 19:1655-1662.
- Chen, X-Y.; Chen, Y.; Heinstein, P.; Davisson, V. J. (1995) Cloning, expression, and characterization of (+)-δ-cadinene synthase: A catalyst for cotton phytoalexin biosynthesis. Archives of Biochemistry and Biophysics 324: 255-266.
- East, N. E.; Anderson, M.; Lowenstine, L. J. (1994) Apparent gossypol-induced toxicosis in adult dairy goats. Journal of the American Veterinary Medical Association 204: 642-643.
- Gadelha, I. C. N.; Fonseca, N. B. S.; Oloris, S. C. S.; Melo, M. M.; Soto-Blanco, B. (2014) Gossypol toxicity from cottonseed products. Scientific World Journal, vol. 2014, Article ID 231635, 11 pages. doi\\10. 1155:2014:231635.
- Haschek, W. M.; Beasley, V. R.; Buck, W. B.; Finnell, J. H. (1989) Cottonseed meal (gossypol) toxicosis in a swine herd. Journal of the American Veterinary Medical Association 195: 613-615.
- Hedin, P. A.; Parrott, W. L.; Jenkins, J. N. (1992) Relationships of glands, cotton square terpenoid aldehydes, and other allelochemicals to larval growth of Heliothis virescens (Lepidoptera, Noctuidae). Journal of Economic Entomology 85: 359-364.
- Henry, M. H.; Pesti, G. M.; Brown, T. P. (2001) Pathology and histopathology of gossypol toxicity in broiler chicks. Avian Diseases 45: 598-604.
- Holmberg, C. A.; Weaver, L. D.; Guterbock, W. M.; Genes, J.; Montgomery, P. (1988) Pathological and toxicological studies of calves fed a high concentration cotton seed meal diet. Veterinary Pathology 25: 147-153.
- Jenkins, J. N.; Maxwell, F. G.; Lafever, H. N. (1966) The comparative preference of insects for glanded and glandless cottons. J. Econ. Entomol. 59:352-356.
- Jonston, C.; Watts, A. B. (1964) Chick feeding value of meals prepared from glandless cottonseed. Poult. Sci. 43:957-963.
- Kenar, J. A. (2006) Reaction chemistry of gossypol and its derivatives. Journal of the American Oil Chemists' Society 83: 269-302.
- Langenheim, J. H. (1994) Higher-plant terpenoids - a phytocentric overview of their ecological roles. J. Chem. Ecol. 20:1223-1280.
- LaRue, D., Knabe, D.; Tanksley, T. D. Jr. (1985) Commercially processed glandless cottonseed meal for starter, grower and finisher swine. J. Anim. Sci. 60:495-502.
- Lukefahr, M. J., Noble, L. W.; Houghtaling, J. E. (1966) Growth and infestation of bollworms and other insects on glanded and glandless strains of cotton. J. Econ. Entomol. 59:817-820.
- Lusas, E. W.; Jividen, G. M. (1987) Glandless cottonseed: A review of the first 25 years of processing and utilization research. Journal of the American Oil Chemists' Society 64: 839-854.
- Maxwell, F. G., Lafever, H. N.; Jenkins, J. N. (1965) Blister beetles on glandless cotton. J. Econ. Entomol. 58:792-793.
- McAuslane, H. J.; Alborn, H. T. (1998) Systemic induction of allelochemicals in glanded and glandless isogenic cotton by Spodoptera exigua feeding. Journal of Chemical Ecology 24: 399-416.
- Morgan, S., Stair, E. L., Martin, T., Edwards, W. C.; Morgan, G. L. (1988) Clinical, clinico-pathologic, pathologic, and toxicologic alterations associated with gossypol toxicosis in feeder lambs. American Journal of Veterinary Research 49: 493-499.
- Randel, R. D., Chase, C. C. Jr.; Wyse, S. J. (1992) Effects of gossypol and cottonseed products on reproduction of mammals. Journal of Animal Science 70: 1628-1638.
- Rathore, K. S., Sunilkumar, G., Stipanovic, R. D.; Wedegaertner, T. C. (2008) RNAi-mediated, selective and substantial reduction in gossypol levels from cottonseed to enhance its food and feed value. In: Proceedings of World Cotton Conference, September 10-14, Lubbock, TX.
- Reid, B., Galaviz-Moreno, S.; Maiorino, P. (1984) A comparison of glandless and regular cottonseed meals for laying hens. Poult. Sci. 63:1803-1809.
- Richardson, C. M., Siccardi, A. J., Palle, S. R., Campbell, L. M., Puckhaber, L., Stipanovic, R. D., Wedegaertner, T. C., Rathore, K. S.; Samocha, T. M. (2016) Evaluation of Ultra-low Gossypol Cottonseed and Regular Glandless Cottonseed Meals as Dietary Protein and Lipid Sources for Litopenaeus vannamei Reared Under Zero- Exchange Conditions. Aquaculture Nutrition 22: 427-434.
- Risco, C. A.; Chase, C. C. Jr. (1997) Gossypol. In: Handbook of Plant and Fungal Toxicants. D’Mello, J. P. F. (Ed. ) Boca Raton, FL: CRC Press. pp. 87-98.
- Roberson, R. (1970) A comparison of glandless cottonseed meal and soybean meal in laying diets supplemened with lysine and methionine. Poult. Sci. 49:1579-1589.
- Sasidhar, P. V. K.; Suvedi, M. (2015) Integrated Contract Broiler Farming: An Evaluation Case Study in India. Modernizing Extension and Advisory Services, USAID. https://agrilinks. org/library/integrated-contract-broiler-farming-evaluation-case-study-india-long.
- Smalley, S. A.; Bicknell, E. J. (1982) Gossypol toxicity in dairy cattle. Compendium on Continuing Education 4: 378-381.
- Stanford, E. E.; Viehoever, A. (1918) Chemistry and histology of the glands of the cotton plant, with notes on the occurrence of similar glands in related plants. Journal of Agricultural Research 13: 419-435.
- Stipanovic, R. D.; Bell, A. A.; Benedict, C. R. (1999) Cotton pest resistance: the role of pigment gland constituents. In: Biologically Active Natural Products: Agrochemicals, Cutler, H. G.; Cutler, S. (Eds. ) CRC Press. Florida. pp. 211-220.
- Sunilkumar, G.; Connell, J. P.; Smith, C. W.; Reddy, A. S.; Rathore, K. S. (2002) Cotton α-globulin promoter: Isolation and functional characterization in transgenic cotton, Arabidopsis, and tobacco. Transgenic Research 11: 347-359.
- Sunilkumar, G.; Campbell, L. M.; Puckhaber, L.; Stipanovic, R. D.; Rathore, K. S. (2006) Engineering cottonseed for use in human nutrition by tissue specific reduction of toxic gossypol. Proceedings of the National Academy of Sciences USA 103: 18054-18059.
- Tilman, D.; Clark, M. (2014) Global diets link environmental sustainability and human health. Nature 515: 518-522
- Waldroup, P.; Keyser, E.; Tollett, V.; Bowen, T. (1968) The evaluation of a low-gossypol glandless cottonseed meal in broiler diets. Poult. Sci. 47:1179-1186.
- World Bank Report number 83177-GLB (2013) FISH TO 2030. Prospects for Fisheries and Aquaculture. http://www. fao. org/docrep/019/i3640e/i3640e. pdf
- Zhang, W-J.; Xu, Z-R.; Pan, X-L.; Yan, X-H.; Wang, Y-B. (2007) Advances in gossypol toxicity and processing effects of whole cottonseed in dairy cows feeding. Livestock Science 111: 1-9.
- Zhang, W-J.; Xu, Z-R.; Pan, X-L.; Yan, X-H.; Wang, Y-B. (2007) Advances in gossypol toxicity and processing effects of whole cottonseed in dairy cows feeding. Livestock Science 111: 1-9.