Feed additive strategies for replacement of antimicrobial growth promoters and a responsible use of antibiotics
L.A. den Hartog1,2, C.H.M. Smits2, W.H. Hendriks1
1 Wageningen University, Animal Nutrition Group, P.O. Box 338, 6700 AH Wageningen, the Netherlands
2Trouw Nutrition Research and Development, P.O. Box 220, 5830 AE Boxmeer, the Netherlands
Abstract
The rapid development of antimicrobial resistance (AMR) in human health care urges the need for effective strategies to reduce antibiotic use in animal production. The Netherlands and Denmark have already implemented successful strategies to reduce antibiotic usage in animal production. Part of the success of the reduction in antibiotic use may be attributed to the wide application of selected feed additives and combinations thereof targeting intestinal microbiota and immunity. Productivity and health responses can be obtained in animals similar to those reported for antimicrobial growth promoters by improving microbiological quality of drinking water and feed, stabilization of the intestinal microbiota and enforcement of the mucosal barrier of the host. Regulatory recognition of the prophylactic effects of feed additives in animal health should further facilitate the progress to reduce AMR.
Introduction
Antimicrobial resistance (AMR) is a present danger and future threat for human as well as animal health. Prophylactic use of antibiotics in human health care and animal production is a key driver for the rapid development of AMR that we are facing nowadays. It has been demonstrated that reduction in antibiotic use may reduce prevalence of AMR pathogens in human and animal populations. This provides us guidance that part of the strategy to fight AMR should be focused on reduction of antibiotic usage (Friedman and Whitney, 2008). With the expected increase in animal protein production globally (Boland et al., 2013), the necessity of responsible use of antibiotics in animal production will be evident.
Antimicrobial consumption is expected to rise by 67% by 2030, and to nearly double in Brazil, Russia, India, China and South Africa if no additional restrictions on their use are taken. Especially the prophylactic use of antibiotics and their application as a growth promotor are currently under pressure in certain countries. The European commission decided to ban all antimicrobial growth promoters (AGP) in 2006. The Netherlands, for example, adopted very strict policies for application of antibiotics. It has led to a significant reduction (58%) in antibiotic usage licensed for prophylactic and therapeutic use from 2009 to 2014 (MARAN, 2015; Figure 1).
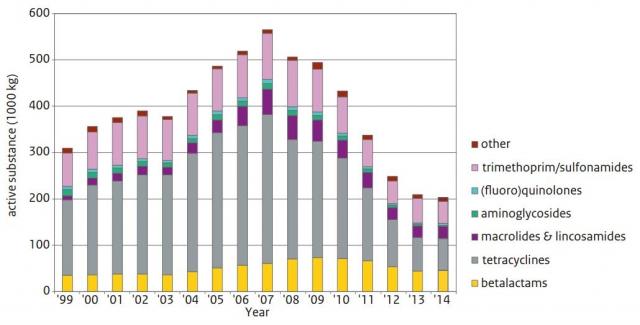
Figure 1: Antimicrobial veterinary medicinal product sales 1999-2014 in livestock in the Netherlands, source MARAN (2015). A significant reduction in antibiotic usage of 58% has been realized from 2009 to 2014.
Stricter biosecurity programmes, a more targeted administration of antibiotics to the animals via drinking water or individual treatment, and well-designed vaccination strategies are examples of best practices implemented by farmers. Besides these measures, various strategies are followed to support animal health via drinking water and/or via the feed. The objective of this contribution is to highlight opportunities and describe the potential contribution of feed additives in programmes aimed at the responsible prudent use of antibiotics.
Feed additive strategies
Antimicrobial growth promoters are still commonly used in most countries outside the EU (van Boeckel et al., 2015). Reported growth-promoting effects differ greatly and are highly dependent of the health status. The growth response to AGPs appears to be small in optimized production systems, suggesting that the economic impacts of a ban on AGPs could be limited in high-income industrialized countries but potentially higher in lower income countries with less developed hygiene and production practices (Laxminarayan et al., 2015).
Similar productivity effects as reported by Laxminarayan et al. (2015) may be obtained with feed additives or combinations that have an impact on microbiota composition and either directly or indirectly modulate the immune system. The ‘toolbox’ here from which the nutritionist can choose consists of a wide range of functional ingredients (Table 1).
Table 1: Examples of functional feed ingredients which can be applied to modulate intestinal microbiota and immunity
|
Products |
In vivo effects |
| Various short- and medium-chain fatty acids (SCFA, MCFA) and other organic acids (OA). | Organic acids are used for preservation, but SCFA, MCFA and OA also exert antimicrobial activity in the gastrointestinal tract and influence microbial activity and diversity (Canibe et al., 2001; FEFANA, 2014, Suryanayarana, 2012; Zentek et al., 2011). The pH lowering effect of acids in the first hours after ingestion of a meal has been reported to contribute to the barrier function of the stomach by preventing colonization of the GIT by pathogens (Hansen et al., 2007). Formate and MCFA have bacteriostatic properties even in relative neutral pH ranges of 6 to 7, which is the pH in the proximal part of the small intestinal tract. |
| Butyrate | Butyrate has pronounced bioactivity in the gut. It enhances proliferation of enterocytes, promotes mucus secretion and may have anti-inflammatory properties (Berni Canani, 2011; Hamer, 2008). In vitro studies have shown that butyric acid also down-regulates the expression of specific virulence genes of Salmonella spp (Gantois et al., 2006). |
| Plant extracts, phytochemicals | A wide range of botanicals (natural botanically defined products according to EU feed additive definitions) or synthetic chemically defined flavourings, also have antimicrobial activity (Burt et al., 2004; Upadhyay et al., 2015; Yang et al., 2009). The gut sensing effects of some of these compounds, however, may be more relevant in relation to gastrointestinal health and immunity of the host. Administration at a relative low dose of some of these compounds (in ppm ranges of <100 ppm) has shown to induce significant changes in mucosal immunity (Furness et al., 2013; Gallois et al, 2009; Vondruskova et al., 2010). The mode of action may consist of stimulation of a wide range of neuro-endocrine and immune-modulatory receptors. |
|
Probiotics |
Probiotics in general may modulate the intestinal microbiota composition and the immune system (Chaucheyras-Durand and Durand, 2010; Ezema, 2013; Vondruskova et al. 2010). Most commonly applied in pigs and poultry are Bacillus spp based probiotics because of their heat stability of spores during pelleting. Another range of probiotics is based on live yeasts. These are mainly applied in dairy nutrition to improve rumen efficiency and prevention of rumen acidosis but also find their application in sow and piglet feed. In newly hatched or newborn animals, ‘starter cultures’, also other bacteria like specific Lactobacilli or Enterococci species, are sometimes applied to steer the initial microbiota in a desired direction. |
|
Prebiotics |
Specific sugars and fibre sources are able to modulate the intestinal microbiota and selectively stimulate specific groups of bacteria who are believed to be beneficial for animal health (Gaggia et al., 2010; Hajati and Rezaei, 2010; Vondruskova et al., 2010; Yang et al., 2009). Some sugars are able to block the binding of pathogens to the mucosa, for example mannose-based sugars can block the binding of some Salmonella spp. to the mucosa (Oyofo et al., 1989) |
| Microbial derived additives from bacteria, yeasts and fungi | The cell walls of yeast contains beta 1,3/1,6 branched glucans for which specific receptors are present in immune cells (macrophages) embedded in the mucosal surface. The mode of action has been extensively studied and reviewed and lately has received more attention in human nutrition and medicine (Rop et al., 2009). Studies indicate that it is possible to improve the immune-competence of young animals with beta-glucans (Saeed et al., 2014). The products are commonly applied in diets for young animals as well as fish and shrimp feed. |
|
Enzymes |
Xylanase is an example of an enzyme that can contribute to health in broiler chickens. Broiler diets with a high level of wheat may induce a high digesta viscosity leading to disturbance of the microbial balance and eventually to maldigestion and malabsorption (Langhout et al., 2000; Smits et al., 1997; Yang et al., 2009). Xylanase reduces the viscous properties of arabinoxylans in wheat. |
|
Additional nutritional effects of vitamins and trace elements |
Some vitamins and trace elements may have an effect on immune-competence of young animals when applied in much higher levels than NRC requirements. However, most vitamins are already supplied in practice at relative high levels above requirements. The role of additional supply of zinc and special forms of zinc has been studied and associated with various health parameters in animal studies, mainly related to immunity and skin condition parameters (Park et al., 2004). The use of high ZnO levels (2500 ppm) in piglet feed for control of post-weaning diarrhea is not in scope here. It is regarded as a prophylactic veterinary measure. |
| Others | The above functional ingredients are the most extensively described in literature. Other alternatives include for example antimicrobial peptides, egg yolk antibodies, rare earth elements and clays, recently reviewed by Thacker (2013) for swine. |
These ‘tools’ include for example short- and medium-chain fatty acids and other organic acids, prebiotic sugars and fibres, probiotics, botanicals with a wide range of plant extracts and microbial derived additives from yeasts and fungi. In general, these tools can be used to modulate the intestinal microbiota and immune system in a specific, desired direction. The general components of the barrier function are illustrated in Figure 2.
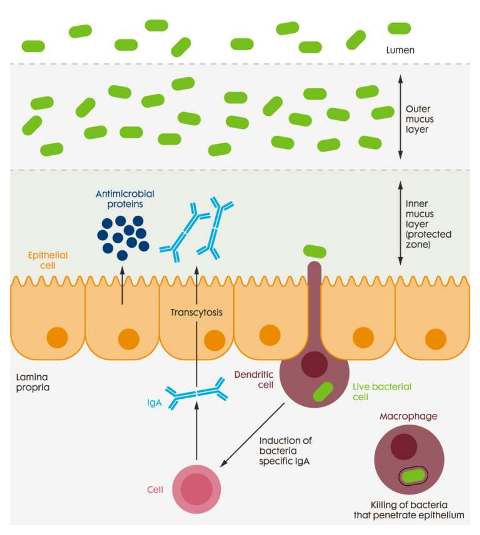
Figure 2 The mucosal barrier is composed of the mucosa associated microbiota, the mucus layer, the gut mucosa and the embedded immune system, all which can be modulated by various feed additives (after Hooper, 2009).
In order to define the appropriate intervention strategy, it is important to have a more detailed understanding of causes of gastrointestinal disorders and disease. The mucosal barrier can be disturbed by various environmental stress factors leading to variation in feed intake and impaired functioning of the gut, which may lead to sudden changes in the microbial balance. A drastic change in microbial balance is often referred to as ‘dysbiosis or dysbacteriosis’ (Teirlynck et al., 2011; Carding et al., 2015). Dysbiosis can be caused by transitions such as weaning, primary infectious challenges, environmental stress, dietary imbalances, or immune incompetence. Dysbiosis is characterized by abnormal changes in microbial counts, activity and changes in microbiota composition and diversity.
A relative effective first route to support the animal with management of its microbiota is mild acidification of water and/or feed to enhance the decrease in pH of the digesta in the stomach of pigs or the crop, proventriculus and gizzard of birds. Organic acids increase the barrier for entry of pathogens and will also support control of bacterial activity in the proximal intestinal tract (Walsh et al., 2007; Suryanayarana et al., 2012; FEFANA, 2014). Water and feed acidification may contribute in this way to maintaining a stable microbiota in broilers and piglets. The efficacy of organic acids can be further enhanced by inclusion of MCFA that exert antimicrobial activity at relative neutral pH ranges and have a higher efficacy towards control of acidophilic bacteria (Awati et al., 2012a,b). Secondly, an important intervention focus could be directed towards strengthening the mucosal barrier function. Butyrate, but also specific plant extracts, may have pronounced effects on the mucosal barrier function by increasing mucus production, epithelial cell proliferation and modulation of the gut associated immune system (Awati et al., 2012c). This, however, will increase nutrient requirement as well as energy requirements due to increased endogenous excretions and activation of the immune system. The latter, however, would likely be a minor ‘cost’, compared to animals with dysbacteriosis where these costs and the subsequent loss of production are significantly higher. Combining feed additives with such different functions and mode of actions is a promising strategy not only to replace AGP, but is also expected to have prophylactic effects.
We recently tested in a series of studies a specific combination of organic acids, with butyrate, MCFA and a selected phenolic compound in both broiler chickens and piglets. The butyrate and MCFA component had controlled-release properties to deliver the bioactive in more distal sections of the gastrointestinal tract. The results of a meta-analysis of the piglet studies are shown in Table 2.
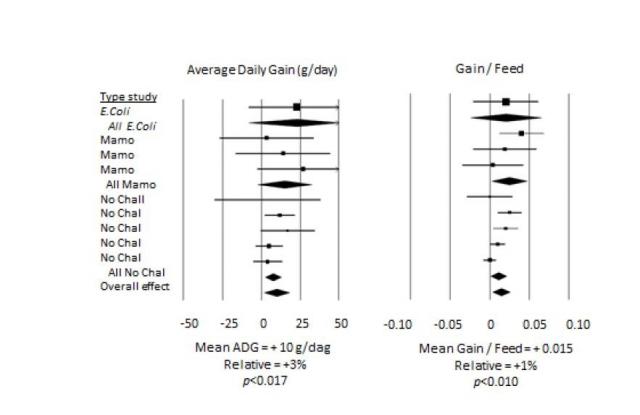
Table 2 Meta-analysis of the response in performance of piglets fed a blend of organic acids, butyrate, medium chain fatty acids and selected phenolic compound (Presan) in 9 studies (unpublished data Trouw Nutrition R&D). The 9 studies represent 1 E.coli challenge study, 3 studies in which the hygiene conditions were suboptimized (MAMO) and 5 studies with no challenge (No Chal). The inclusion level of the blend was 0.1 or 0.2%.
On average, a significant improvement in average daily gain and feed efficiency was observed of respectively 3 and 1%. The diarrhea incidence in the piglet studies (in total 5 studies could be used for this analysis) tended to be lower in piglets fed the additive blend (p < 0.056). These average effects are in
line with the magnitude of the responses described by Laxminarayan et al. (2015) for AGP. In challenge conditions, the effect of the feed additive FA blend on average daily gain and feed efficiency was more pronounced, demonstrating indirectly that this intervention strategy may, at least partially, prevent or ameliorate the possible effect of infectious challenges. Such combination concepts should not be seen as ‘curative’ but may contribute to the prophylaxis of specific enteric diseases and disorders. In the above mentioned blend, probiotics or prebiotics were not applied, but also these additives may be used as effective tools for creating synergistic blends to support gastrointestinal health. However, we observed less consistent results in experiments with specific added probiotics and prebiotics in earlier research (unpublished data).
Overall, the stability of the intestinal microbiota and a strong mucosal barrier are key targets of feed additives to realize the desired effects in productivity and health. Stability can be reached by (mild) antimicrobial activity without disturbing the microbial balance. A strong mucosal barrier can be achieved by enforcing gut integrity and modulate the immune system in such a way that the response is adequate but not excessive (inflammation).
The global feed industry can play a major role by adopting new insights and novel technologies in feed formulations and feed additives. The speed at which this can be implemented will be important for the success to combat AMR. In this context it may be important to note that regulatory institutions should facilitate the rapid adoption of new insights and technologies and create a regulatory space in which it is possible to claim health effects of dietary measures, that are supported by science but not (yet) recognized from a legal point of view.
References
Awati, A., Smits, C.H.M., Roubos-van den Hill, P., Timmermans, H. (2012a) Medium chain fatty acids and organic acid mixture improves performance by preventing microbial overgrowth and improve microbial diversity in the jejunum of broiler chickens. Abstract, XXIV Worlds Poultry Congress, WPSA, Salvador, Bahia, Brazil.
Awati, A., Smits, C.H.M., Timmermans, H.M. (2012b) Medium chain fatty acids and organic acid based feed additives improve animal performance and reduce bacterial overgrowth in the small intestine of weaning piglets. Abstract 12th Symposium Digestive physiology in pigs, Keystone, Colorado, USA.
Awati, A., Santos, R.R., Smits, C.H.M., Fink-Gremmels, J. (2012c) Protective effects of feed additives on gut barrier function and systemic host defense in chickens exposed to heat stress. Abstract, XXIV Worlds Poultry Congress, WPSA, Salvador, Bahia, Brazil.
Berni Canini, R., Di Constanzo, M., Leone, L., Pedata, M., Meli, R., Calignano, A. (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World Journal of Gastroenterology 17: 1519-1528.
Boland, M. J., Rae, A. N., Vereijken, J. M., Meuwissen, M. P. M., Fischer, A. R. H., van Boekel, M. A. J. S., Rutherfurd, S. M., Gruppen, H., Moughan, P. J. and Hendriks, W. H. (2013) The future supply of animal-derived protein for human consumption. Trends in Food Science and Technology 29: 62-73.
Burt, S. (2004) Essential oils: their antibacterial properties and potential applications in foods – a review. International Journal of Food Microbiology 94: 223-253.
Canibe, N., Steien, S.H., Overland, M., Jensen, B.B. (2001) Effect of K-diformate in starter diets on acidity, microbiota, and the amount of organic acids in the digestive tract of piglets, and on gastric alterations. Journal of Animal Science 79: 2123-2133.
Carding, S., Verbeke, K., Vipond, D.T., Corfe, B.M., Owen, L.J. (2015) Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health & Disease, 26: 26191.
Chaucheryas-Durand, F., Durand, H. (2010) Probiotics in animal nutrition and health. Beneficial microbes 1: 3-9.
Ezema, C. (2013) Probiotics in animal nutrition: A review. Journal of Veterinary Medicine and Animal Health 5: 308-316.
FEFANA Publication (2014) Organic acids in animal nutrition.
Friedman, C.R., Whitney, C.G. (2008) It’s time for a change in practice: Reduce in antibiotic use can alter antibiotic resistance. Journal of Infectious Diseases 197: 182-283.
Furness, J.B., Rivera, L.R., Cho, H-J., Bravo, D.M., Callaghan, B. (2013) The gut as a sensory organ. Nature Reviews Gastroenterology and Hepatology 10: 729-740.
Gaggia, F., Mattarelli, P., Biavati, B. (2010) Probiotics and prebiotics in animal feeding for safe food production. International Journal of Food Microbiology 141: S15-S28.
Gallois, M., Rothkotter, H.J., Bailey, M., Stokes, C.R., Oswald, I.P. (2009) Natural alternatives to in-feed antibiotics in pig production: can immunomodulators play a role? Animal 3-12: 1644-1661,
Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Hautefort, I., Thompson, A., Hinton, J., van Immerseel, F. (2006) Butyrate specifically decreases Salmonella pathogenicity Island I gene expression. Applied and Environmental Microbiology 72: 946-949.
Hajati, H., Rezaei, M. (2010) The application of prebiotics in poultry production. International Journal of Poultry Science 9: 298-304.
Hamer, H.M., Jonkers, D., Venema, K., Vanhoutvin, S., Troost, F.J., Brummer, R.-J. (2008) Review article: the role of butyrate on colonic function. Alimentary Pharmacology & Therapeutics 27: 104-119.
Hansen, C.F., Riis, A.L., Bresson, S., Hojberg, O., Jensen, B.B. (2007) Feeding organic acids enhances the barrier function against pathogenic bacteria of the piglet stomach. Livestock Science 108: 206-209.
Hooper, L.V. (2009) Immune mechanisms that confine intestinal bacteria to the lumen. Nature Reviews Microbiology 7: 367-374.
Langhout, D.J., Schutte, J.B., de Jong, J., Sloetjes, H., Verstegen, M.W., Tamminga, S. (2000) Effect of viscosity on digestion of nutrients in conventional and germ-free chicks. British Journal of Nutrition 85: 533-540.
Laxminarayan, R., van Boeckel, T., Teillant, A. (2015) The economic costs of withdrawing antimicrobial growth promoters from the livestock sector. OECD Food Agriculture and Fisheries Papers, no. 78, OECD publishing.
MARAN (2015) Monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands in 2014.
Oyofo, B.A., DeLoach, J.R., Corrier, D.E., Norman, J.O., Ziprin, R.L., Mollenhauer, H.H. (1988) Prevention of Salmonella typhimurium colonization of broilers with D-mannose. Poultry Science 68: 1357-1360.
Park, S.Y., Birkhold, S.G., Kubena, L.F., Nisbet, D.J., Ricke, S. (2004) Review on the role of dietary zinc in poultry nutrition, immunity and reproduction. Biological Trace Element Research 101 (2): 147-163.
Rop, O., Mlcek, J., Jurikova, T. (2009) Beta-glucans in higher fungi and their health effects. Nutrition reviews, 67: 624-631.
Saeed et al. (2014) Epigenetic programming of monocyte-macrophage differentiation and trained innate immunity. Science 345, 1251086.
Smits, C.H.M., Veldman, A., Verstegen, M.W.A,, Beynen, A.C. (1997) Dietary carboxymethylcellulose with high instead of low viscosity reduces macronutrient digestion in broiler chickens. Journal of Nutrition 127: 483-487.
Suryanayarana, M.V.A.N., Suresh, J., Rayasekhar, M.V. (2012) Organic acids in swine feeding: A review. Agricultural Science Research Reviews. 2(9): 523-533.
Teirlynck, E., Gussem, M.D.E., Dewulf, J., Haesebrouck, F., Ducatelle, R., van Immerseel, F. (2011) Morphometric evaluation of ‘dysbacteriosis’ in broilers. Avian Pathology 40:2; 139-144.
Thacker, P.A. (2013) Alternatives to antibiotics as growth promoters for use in swine production: a review. Journal of Animal Science and Biotechnology 4: 35.
Upadhyay, A. Upadhyaya, I., Kollanoor-Johny, A., Venkitanarayanan, K. (2014) Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Hindawi Publishing Corporation, BioMed Research International, article ID 761741, 18 p.
Van Boeckel, T.P, Brower, C., Gilbert, M, Grenfell, B.T., Levin, S.A., Robinson, T.P., Teillant, A., Laxminarayan, R.,. (2015) Global trends in antimicrobial use in food animals. PNAS Early Edition, p1-6. www.pnas.org/cgi/doi/10.0173/pnas. 1503141112
Vondruska, H., Slamova, R., Trckova, M., Zraly, Z., Pavlik, I. (2010) Alternatives to antibiotic growth promoters in prevention of diarrhea in weaned piglets: a review. Veterinarni Medicina, 55: 199-224.
Walsh, M.C., Sholly, D.M., Hinson, R.B., Saddoris, K.L., Sutton, A.L., Radcliffe, J.S., Odgaard, R., Murphy, J., Richert, B.T. (2007) Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. Journal of Animal Science 85: 1799-1808.
Yang, Y., Iji, P.A., Choct, M. (2009) Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poultry Science Journal 65: 97-113.
Zentek, J., Buchheit-Renko, S., Ferrara, F., Vahjen, W., Van Kessel, A.G., Pieper, R. (2011) Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets, Animal Health Research Reviews 12: 83–93.

